Tianjin University's Zhe Sun Research Group Publishes in JACS: Unique Cyclization Reaction Based on Singlet Diradicals for Constructing Nanographene Molecules
In organic reactions, radical species typically exist as intermediates that cannot be isolated. However, with advances in synthetic chemistry, a series of stable organic diradical and polyradical compounds have been synthesized and isolated. Due to their open-shell electronic structure, these compounds often exhibit unique reactivity in cyclization reactions, which can be used to synthesize conjugated large π-electron molecules. For example, Kubo and Juricek have reported that the 6π electrocyclization reaction of singlet diradicals can proceed via a thermally forbidden pathway, bypassing the Woodward-Hoffmann rules. Similarly, Jishan Wu's team reported an intramolecular radical coupling reaction from a tetraradical precursor to construct near-infrared laser materials based on nanographene molecules. However, compared to traditional cyclization reactions based on closed-shell systems (e.g., Scholl reactions), the use of open-shell electronic precursors to construct functional nanographene molecular materials remains limited. Key challenges include synthesizing stable open-shell systems and regulating their reactivity to broaden the application of such reactions.
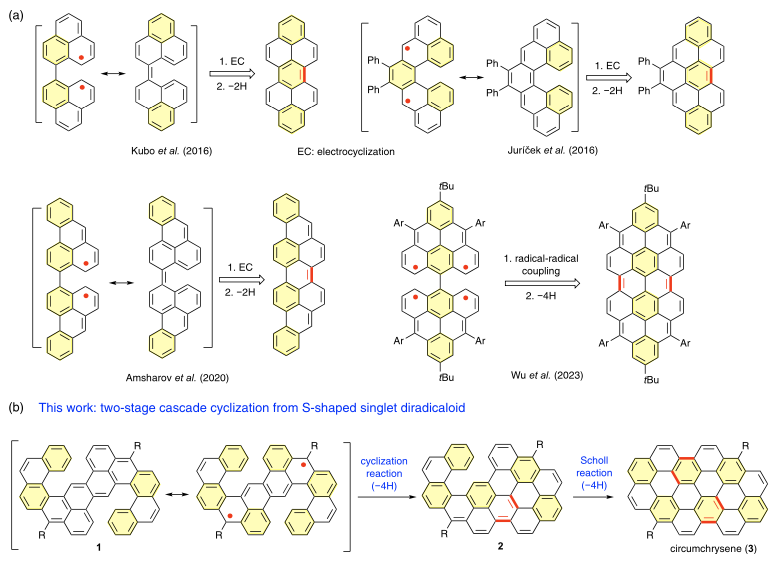
The research group of Zhe Sun from Tianjin University focuses on developing new reactions (e.g., Angew. Chem. Int. Ed. 2023, Nat. Synth. 2023) and materials based on organic conjugated radicals (Adv. Mater. 2023, J. Am. Chem. Soc. 2022). Recently, in collaboration with the research group of Chen Guang from Shaanxi University of Science and Technology, they designed and synthesized an S-shaped double helicene compound (1) with a singlet diradical ground state. Due to its diradical nature, 1 lowers the energy barrier of the thermally induced electrocyclization reaction, allowing it to efficiently undergo a cascade cyclization reaction at the helicene position, forming two C-C bonds. In the presence of an oxidizing agent, dehydrogenation occurs to yield a conjugated extended helicene compound (2). Since 2 has a closed-shell electronic structure, further electrocyclization is not possible. However, using Scholl reaction conditions (DDQ + TfOH), subsequent cyclization reactions were achieved, leading to the synthesis of circumchrysene, one of the largest synthesized circumarene structures to date.
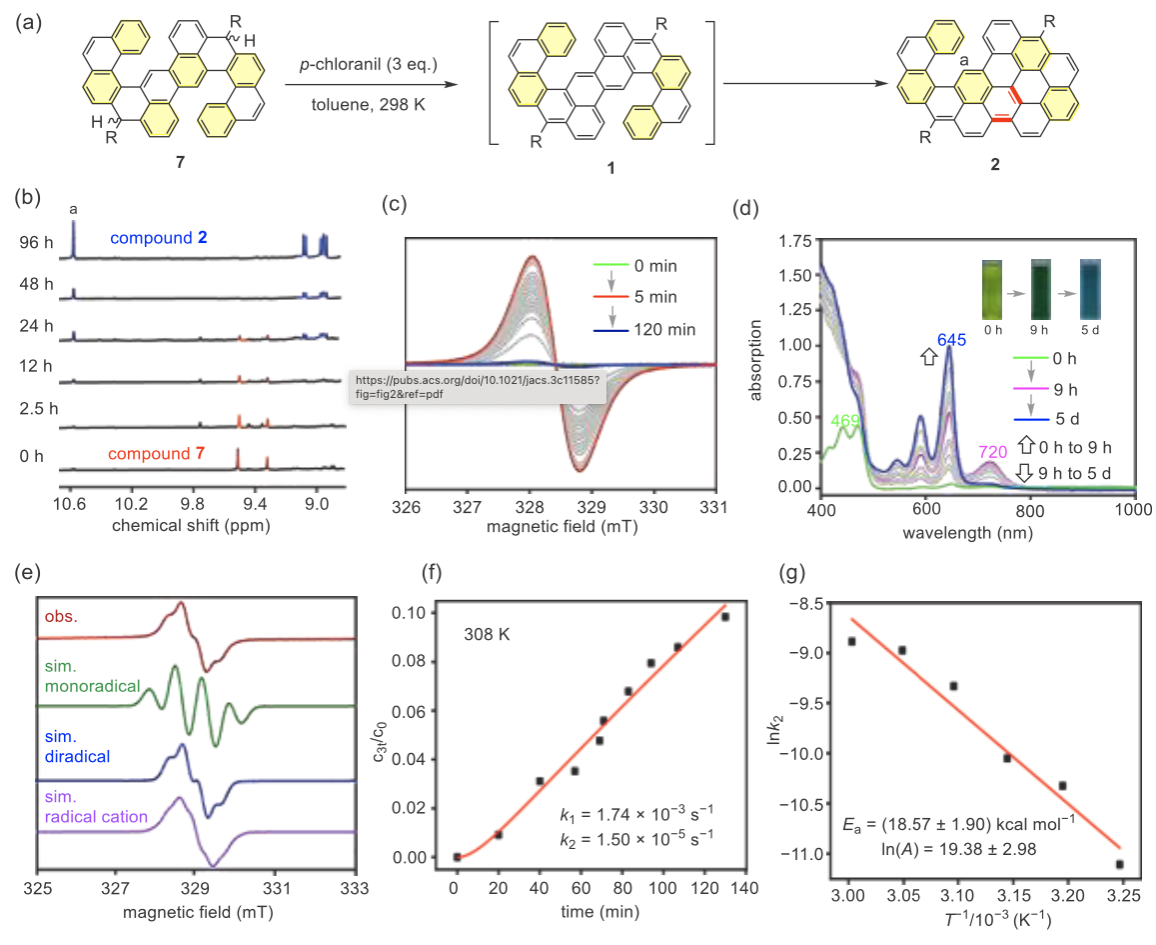
To investigate the mechanism of the cyclization-dehydrogenation step and capture diradical intermediates, the authors conducted in-situ monitoring of the reaction from 7 to 2 using NMR, EPR, and UV-visible-near-infrared spectroscopy. NMR spectra clearly showed the evolution from 7 to 2. After adding tetrachlorobenzoquinone to a toluene solution of 7, an EPR signal was observed within 5 minutes, indicating the formation of paramagnetic species, which gradually disappeared after 2 hours, suggesting full conversion to diamagnetic compounds. The EPR signal intensity decreased with lower temperatures, confirming that the intermediate species was a singlet diradical. UV-visible-near-infrared spectroscopy showed that a long-wavelength intermediate (pink spectrum) appeared during the first 9 hours of the reaction, corresponding to the diradical structure of 1. Theoretical calculations further supported that the singlet pathway was the most energetically favorable. Overall, the authors concluded that the cascade cyclization reaction proceeded through a singlet diradical intermediate. However, the possibility of a cationic radical intermediate cannot be entirely ruled out by the current experimental and theoretical results.
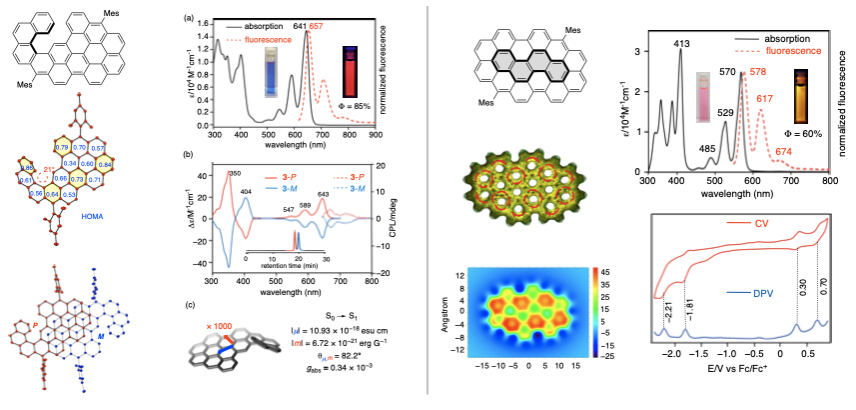
The authors characterized the structure and properties of the resulting compounds 2 and 3. X-ray diffraction analysis of single crystals of 2 revealed a [5]helicene structure with a helical twist angle of 21°. In the crystal, a pair of enantiomers were observed, stacked together through π-π interactions with an average distance of 3.50 Å. DFT calculations showed an enantiomerization barrier of 26 kcal mol−1, slightly higher than that of [5]helicene (24 kcal mol−1). The enantiomers were separated using chiral HPLC, and in solution, 2 exhibited excellent photoluminescent properties with a quantum yield of up to 85%. Its circularly polarized luminescence (CPL) extended into the near-infrared region (646-745 nm), with a brightness (B) of 6.05 M-1 cm-1, making it one of the brightest near-infrared CPL materials. The cyclized product 3 displayed unique electronic structures characteristic of nanographene molecules, along with excellent luminescence and multi-step redox properties, suggesting potential applications in organic electronic and luminescent materials.
This work demonstrates that open-shell conjugated systems as reaction precursors can provide unique reactivity, enabling the synthesis of complex conjugated systems that are challenging to achieve through traditional methods. This opens new avenues for the preparation of functional organic electronic materials and chiral luminescent materials.
The article was published in the Journal of the American Chemical Society (JACS), and the first author is PhD candidate Jinlian Hu from Tianjin University's Institute of Molecular Plus.